The Larese-Casanova Research Group
Research
Oxygen isotopic indicators of selenium biogeochemical processing
PIs: Philip Larese-Casanova, Annalisa Onnis-Hayden
Funding: NSF Environmental Engineering
Selenium pollution of surface waters and sediments is pervasive in the western U.S. due to erosion, mining, combustion, petroleum processing, and irrigation activities. Environmental management strategies that involve monitoring Se fluxes in the water column could benefit from an improved conceptualization of Se biogeochemical processes and new Se characterization methods that directly indicate these processes. We are developing a stable oxygen isotopic approach to clarify reaction pathways for selenium oxyanions and reveal prevailing transformation processes occurring in surface waters, sediments, and engineered remediation systems. Specific objectives are (1) to describe and distinguish sources of oxygen incorporation during biotic and abiotic Se oxidation to selenate, (2) to develop isotope indicators that distinguish and quantify biotic reductive precipitation, abiotic reductive precipitation, and biotic reductive methylation and volatilization associated with uptake, and (3) to improve conceptual models of oxyanion sorption to metal oxides by probing interfacial oxygen exchange mechanisms between oxyanions, oxides, and water molecules that occur during surface complexation formation.
Evaluation of commercial graphene particles for sorption of organic pollutants and organic matter
PI: Philip Larese-Casanova
Funding: Northeastern University
Endocrine disrupting compounds (EDCs) and pharmaceuticals produce adverse effect on the reproductive, neurological and immune systems of human health and wildlife. The occurrence of trace amounts of pharmaceuticals and EDCs have been reported in surface water and even in drinking water for decades. Graphene is a two-dimensional material consisting of a single layer of pure carbon, which has extraordinary capacity to sorb complex organic compounds. We are studying commercially available graphene particles, of varied particle sizes, surface areas, and surface chemistries, for their abilities to sorb EDCs, pharmaceuticals, and organic matter. Graphene may offer a greater specific surface area and a more favorable interaction with low molecular weight organic compounds compared to conventional carbon-based sorbents.
Fate of quantum dots in aquatic environments
PI: Philip Larese-Casanova
Funding: NSF CAREER, Environmental Health & Safety of Nanotechnology
We are exploring explores the behavior of quantum dots—semiconducting nanocrystals with extraordinary electrical properties featured in the next generation of consumer electronics and solar cells— within the environment upon their accidental release from product disposal or industrial waste streams. With the projected market volume for nanomaterial-based products approaching trillions of dollars annually, handling of these products and their waste will inevitably pose challenges to environmental health, particularly for metallic nanomaterials that may be toxic to aquatic organisms. Laboratory experiments will address how quantum dots change physically and chemically within lakes, rivers, and groundwater. The results are expected to identify important chemical reaction processes and to improve analytical chemistry methods for detection and quantification of potentially toxic quantum dots in water.
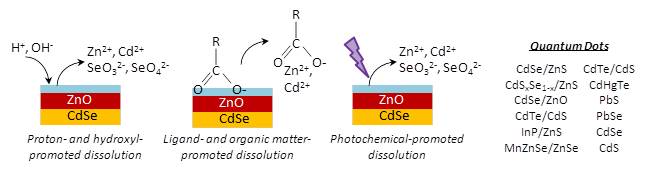
Modes of quantum dot dissolution.

Suspension of hematite (a-Fe2O3), an iron oxide commonly found within groundwater environments capable of facilitating Fe(II)-Fe(III) redox reactions among dissolved Fe(II) cations, Fe(III)-reducing bacteria, and metallic and organic contaminants.